Industries
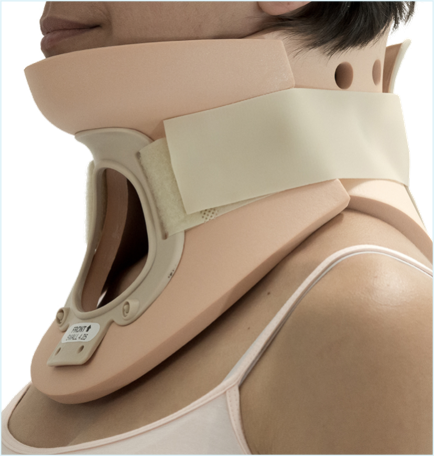
Medical
Why Choose Zotefoams Materials?
- Closed cell structure – will not take in water.
- Non-toxic and hypoallergenic – good for frequent skin contact
- Radiolucent – ideal for MRI, CT, and X-ray applications
- Easy to clean and sterilise – safe with warm water, detergents, and hypochlorite
- Light in weight but strong – comfortable and durable long-term wear
- Good mouldability – allows “direct-to-body” customisation
- Homogeneous and stable – reproducible manufacture and extended-term performance
Zotefoams materials for Medical Applications
Regulatory Support & Biocompatibility
Zotefoams’ materials have been tested to support customer compliance with medical standards including:
- Cytotoxicity (ISO 10993-5)
- Sensitisation (ISO 10993-10)
- Irritation (ISO 10993-23)
- Chemical characterisation (ISO 10993-18)
While we do not use medical-grade resins or manufacture in cleanrooms, our nitrogen-expanded foams are among the purest available. We support customers by providing material data and biocompatibility summaries to help meet MDR and FDA regulatory requirements.
Technical Support
Application Engineering Support
Our experienced Application Engineering team works closely with customers to optimise material selection, design, and processing. From concept to commercialisation, we provide tailored technical support to help you get the best performance from Zotefoams materials in your application.
What makes Zotefoams unique?
Three-Stage Manufacturing
Our unique nitrogen expansion manufacturing process delivers pure, uniform and durable materials.
Sustainability
Our technology and efficient use of raw materials allows us to develop lighter more durable products.